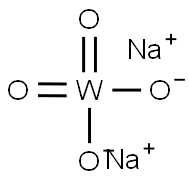TUNGSTEN:Application and Toxicity Studies
GeneralDescription
Tungsten is the bioelement with the highest atomic number 74, and the only bioelement in the third transition row of the periodic table.Tungsten is widely distributed in biology, however, it is not a universal bioelement, the majority of the tungsten-containing enzymes purified to date, originates from anaerobic archaea and bacteria.[1]Tungsten is a heavy metal with several unique physical and mechanical properties such as high melting/boiling points and a density of 19.1 g cm-3. Tungsten occurs naturally in soils and sediments usually in small concentrations; average concentration values in the lithosphere are in the 0.2–2.4 mg kg-1 range.[2]For some species tungsten is essential: their life depends on the presence of the element; for other species tungsten is a facultative bioelement: they choose to make biological use of the element when they experience specific environmental constraints; for the remaining species tungsten is biochemically indifferent or possibly xenobiotic. In light of the aqueous chemistry of tungsten,the entry point of W-biochemistry is a stable, redox indifferent, highly soluble oxoanion; and it has a Mo congener of very similar properties.[1]Tungsten is usually found as oxo-rich tungstate minerals [W(VI)], either as scheelite (CaWO4) or wolframite ([Fe/Mn]WO4), whereas the more reduced tungstenite [WS 2, W(IV)] is very rare.[6]
Tungsten deposits usually occur within, or near to, orogenic belts resulting from subduction-related plate tectonics.Most tungsten is mined from sub-surface (or underground) mines. Tungsten is used and traded in a variety of forms. The unique range of properties of tungsten makes it an essential component in a wide range of products for many applications.[3]The element tungsten is one of the so-called“less-common metals”and occurs in the periodic system in group VI with chromium (atomic number 24) 8 and molybdenum(atomic number 42). In all its properties it closely resembles the latter element. In the pure metallic state tungsten has a lustre somewhat like that of steel.[4]

Figure 1 Powder of the Tungsten
Application
The applications of metallic tungsten are mainly based on its high melting point, high strength at high temperatures, resistance to wear and good conductivity for electricity and heat.Apart from its use as a filament in incandescent lamps, as electrodes for discharge tubes, and for rocket motor nozzles and space vehicles,tungsten is used for electrical contacts, e.g. for interruptors for sparking coils in internal combustion motors for which purpose all the above-mentioned properties are important. In the laboratory many high-temperature applications of tungsten are known, e.g. wires and tubes for heating elements in furnaces with a protective atmosphere, boats
and strips, and thermocouples for temperatures above 2000°C in nonoxidizing atmospheres.Because of its high atomic number and refractory properties tungsten is used for X-ray cathodes, e.g. in medical rotating-anode X-ray tubes.In the powder form tungsten may act as a catalyst in hydrogenation processes.Often alloys with a high tungsten content are used for the same purposes as pure tungsten, because favourable properties may be obtained by alloying or adding other elements or compounds. Special steels containing only a small amount of tungsten and alloys like stellites consume a large part of the world production of tungsten. Tungsten is widely used in the commercial and military ammunition because it has the second highest melting temperature of any element.[5]
Toxicity
1.Impact on human health:The potential involvement of tungsten in the development of cancers and other deleterious health problems. It is also notable that chronic exposure to tungsten, even at very low concentration, is important, probably more so than acute toxicity.[5]In general, toxicity depends on the chemical form of the tungsten compounds and the exposure pathway. Tungsten is transported through the body by blood and it affects the liver, skeleton, kidneys, spleen and other organs. On the one hand,Tungsten became a contaminant and has toxic and can effect health.Compared with leukemic case subjects and non-leukemic comparison subjects, the result showed that leukemic case subjects have high levels of tungsten in their bodies.Using military-grade heavy metal tungsten alloys (90% tungsten) pellets,the high-dose (20 pellets) can developed extremely aggressive tumors,and the low-dose (4pellets) tungsten can also developed tumors, characterized as high-grade pleomorphic rhabdomyosarcomas, which rapidly metastasized to the lungs. In general, metal implants can potentially have carcinogenic effects, but tungsten pellets are more severe than a generic effect from implanting metal.On the other hand, the pre- and post-natal exposure to sodium tungstate may produce subtle neurobehavioral effects in offspring related to motor activity and emotionality.[2]
2.Impact on the ecological environment:Dissolution of tungsten powder significantly acidifies soils. Tungsten powder mixed with soils at rates higher than 1% on a mass basis, trigger changes in soil microbial communities resulting in the death of a substantial portion of the bacterial component and an increase of the fungal biomass. It also induces the death of red worms and plants. These effects appear to be related with the soil acidification occurring during tungsten dissolution. Dissolved tungsten species significantly decrease microbial yields by as much as 38% for a tungsten media concentration of 89 mg l-1. Soluble tungsten concentrations as low as 105mgl-1, cause a decrease in biomass production by 8% which is possibly related to production of stress proteins. Plants and worms take up tungsten ions from soil in significant amounts while an enrichment of tungsten in the plant rhizosphere is observed. Similar tungsten concentrations resulted in the death of ryegrass plants.Evidently, for soils with low tungsten contents the tungsten metal must be distributed in the form of fine powders to impart toxicity in soil microbial communities. As the tungsten content increases even coarser fragments are capable of producing a total toxicity effect.[2]
Reference
[1]Bevers LE, Hagedoorn P L, Hagen W R. The bioinorganic chemistry of tungsten[J]. Coordination Chemistry Reviews, 2009, 253(3-4): 269-290.
[2]Strigul N, Koutsospyros A, Arienti P, et al. Effects of tungsten on environmental systems[J]. Chemosphere, 2005, 61(2): 248-258.
[3]Brown T, Pitfield P. Tungsten[J]. Critical metals handbook, 2014: 385-413.
[4]Rieck G D. Tungsten and its Compounds[M]. Elsevier, 2013.
[5]Witten M L, Sheppard P R, Witten B L. Tungsten toxicity[J]. Chemico-biological interactions, 2012, 196(3): 87-88.
[6]Kletzin A, Adams M W W. Tungsten in biological systems[J]. FEMS Microbiology Reviews, 1996, 18(1): 5-63.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium tungstate manufacturers

US $1.00/KG2025-04-21
- CAS:
- 13472-45-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $30.00-10.00/KG2025-04-15
- CAS:
- 13472-45-2
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg